Descripción
Lexyal Grand is a very dense dermal filler, which is primarily utilized to:
- Fill deep to very deep wrinkles and folds
- Lifting sagging dermal tissues, particularly in the cheek aera
- Contour the facial oval (jawline)
- Sculpt facial features (chin, nose and cheekbones)
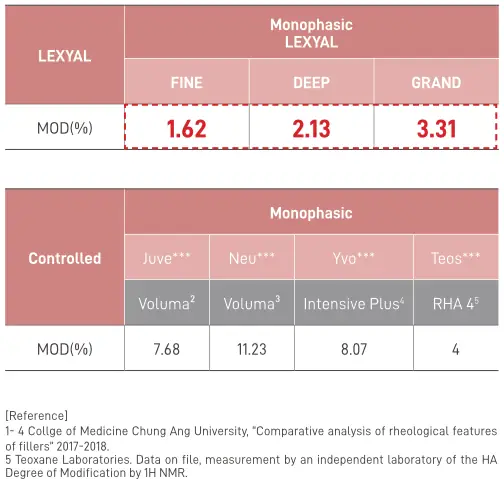
This dermal filler is very safe. Like the other two entries of the Lexyal series, it is CE certified and adherent and is manufactured using the Preserved Natural Cross‑Linked. PNCL™ allows to maintain the natural properties of Hyaluronic acid with Ha purity of over 95%. Once the gel is manufactured, the BDDE levels are below detectable levels – sub 0.2 PPM. The concentration of HA in the gel, itself, is 24mg/ml and the gel has a Modifcation Degree (MoD) of 3.31% – higher than the other two products, but still well below the industry averages. What this means for the filler is that its gel very highly biocompatible and blends with dermal tissues excellently, balancing between supportive properties and the necessary flexibility.
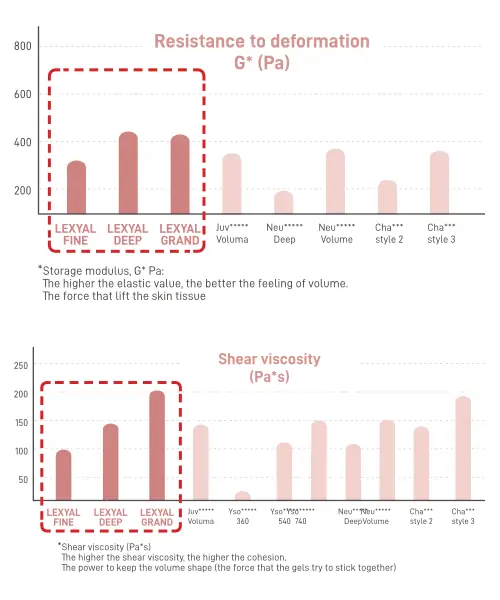
Structural lifting of the Lexyal Grand is excellent, it has tuned viscoelasticity and high G’ and shear viscosity. This allows the shape of the facial feature or the contour of the oval to be properly retained, as well as the lifting effect to remain in place for a longer period. All of this is achieved while keeping the endotoxin levels to a minimum – less than 0.005EU/ml, which is a lot lower than what is accepted as safe levels in the filler industry.
In practice, Lexyal Grand offers facial contouring, filling and lifting for over 12 months, very natural to look at results and, most importantly, a safe experience with minimal post procedure adverse reactions, particularly in regard to inflammation. Each box contains one filler or 1ml and two 27G needles. It is the sculpting product that dermatologists and licensed practitioners chose when then need to fine tune and correct the facial contour.
Key Features
- Usage: Very deep to severe wrinkles, contouring, lifting, sculpting
- HA concentration.: 24 mg/ml
- BDDE residue levels: <0.2 PPM
- MoD: 3.31%
- Endotoxin levels: <0.005 EU/ml
- Patented technology: PNCL™ monophasic stabilization
- Box content: 1 ml pre-filled syringe with dual 27G needles
- Physical properties: High resistance (G’) for structural lift
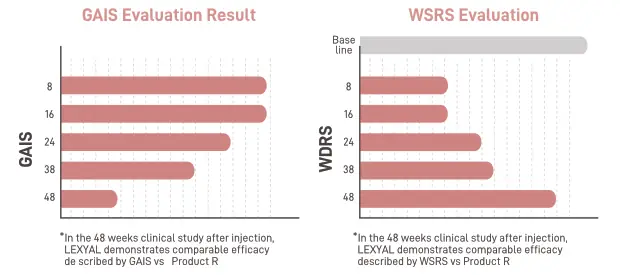
- Clinical Test Data: Proven GAIS/WSRS improvement at 48 weeks
- Certification: CE-marked